
|
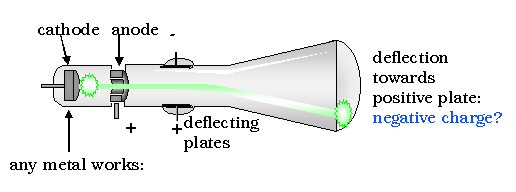
The next great step forward in the understanding of atoms was accomplished by John Thomson. Using a cathode ray scope, Thomson determined that all matter, whatever its source, contains particles of the same kind that are much less massive than the atoms of which they form a part. They are now called electrons, although he originally called them corpuscles. |

.. |
.. His discovery was the result of an attempt to solve a long-standing controversy regarding the nature of cathode rays, which occur when an electric current is driven through a vessel from which most of the air or other gas has been pumped out. |
.. |
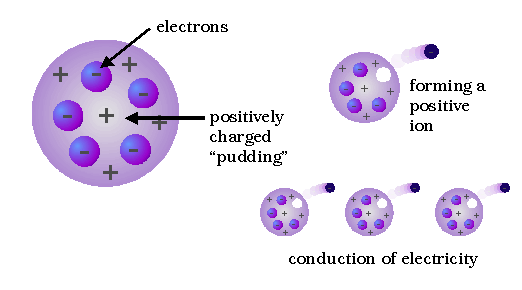
.. By applying an improved vacuum technique, Thomson was able to put forward a convincing argument that these rays were composed of particles. Furthermore, these rays seemed to be composed of the same particles, or corpuscles, regardless of what kind of gas carried the electric discharge or what kinds of metals were used as conductors. From these ideas he developed the idea that atoms are made of negative electrons embedded in a gel of positive charge (a "plum pudding" model). |
.. |
.. Thomson's conclusion that the corpuscles were present in all kinds of matter was strengthened during the next three years, when he found that corpuscles with the same properties could be produced in other ways; e.g., from hot metals. Thomson may be described as "the man who split the atom" for the first time, although "chipped" might be a better word, in view of the size and number of electrons. |
|
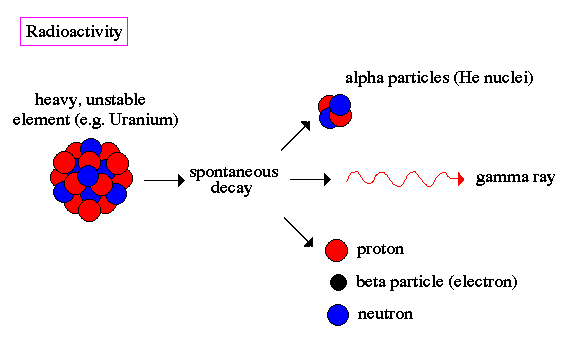
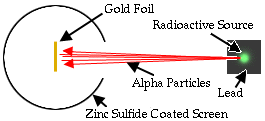
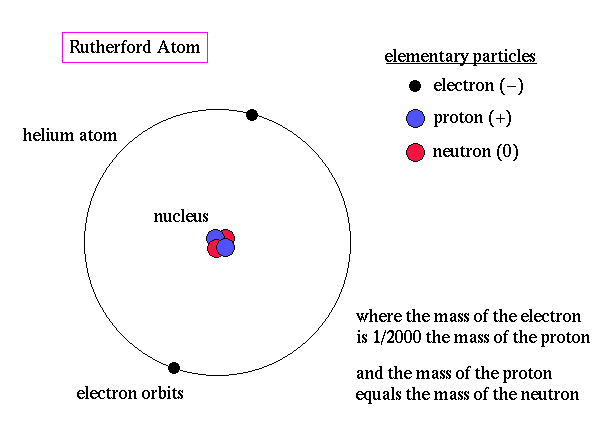
Ernest Rutherford is considered the father of nuclear physics. Indeed, it could be said that Rutherford invented the very language to describe the theoretical concepts of the atom and the phenomenon of radioactivity. Particles named and characterized by him include the alpha particle, beta particle and proton. Rutherford overturned Thomson's atom model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny, massive nucleus. |

.. |
.. By the turn of the 20th century, physicists knew that certain elements emitted fast moving particles of two flavors, alpha particles and beta particles. These elements were typically very heavy (i.e. their atom nuclei were massive) such as uranium and radium. Today we know that heavy nuclei are unstable and `decay', meaning that they spontaneously split into smaller nuclei and emit stray particles. This is called radioactivity. |
.. |
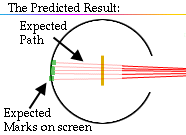
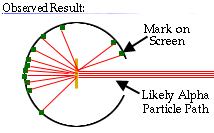
.. The alpha particle was heavy and positively charged, we now know that it is the helium nuclei (2 protons and 2 neutrons). The beta particle was light and negatively charged, the electron. Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. His experiment looked like the following: |

.. |
.. 
|
.. |
.. |
.. |
.. 
|
.. |
.. 
|
.. |
.. |
.. |
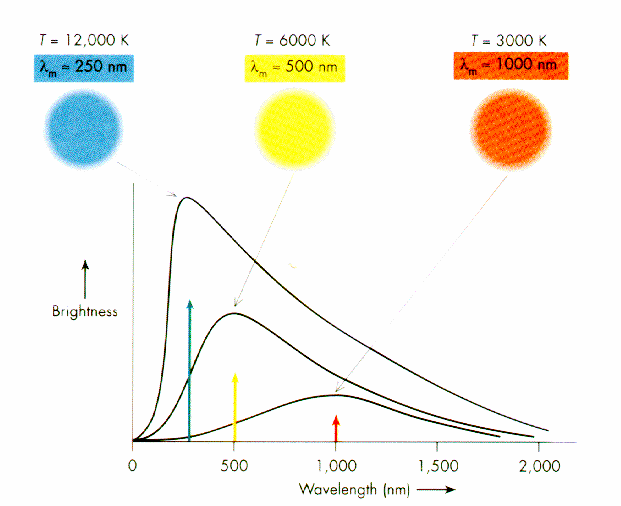
.. Parallel to efforts to understand the inner workings of the atom was research in the late-1800's to understand how matter emits energy. The energy an object emits as a function of the wavelength of light is called its continuous spectrum. The field of science that studies spectrum is called spectroscopy. One of the primary results from the field of spectroscopy was the discovery that the spectrum of an object changes with temperature. .. The Austrian physicist Josef Stefan found in 1879 that the total radiation energy per unit time emitted by a heated surface per unit area increases as the fourth power of its absolute temperature T (Kelvin scale). This means that the Sun's surface, which is at T = 6,000 K, radiates per unit area (6,000/300)4 = 204 = 160,000 times more electromagnetic energy than does the same area of the Earth's surface, which is taken to be T = 300 K. In 1889 another Austrian physicist, Ludwig Boltzmann, used the second law of thermodynamics to derive this temperature dependence for an ideal substance that emits and absorbs all frequencies. Such an object that absorbs light of all colors looks black, and so was called a blackbody. .. The wavelength or frequency distribution of blackbody radiation was studied in the 1890s by Wilhelm Wien of Germany. It was his idea to use as a good approximation for the ideal blackbody an oven with a small hole. Any radiation that enters the small hole is scattered and reflected from the inner walls of the oven so often that nearly all incoming radiation is absorbed and the chance of some of it finding its way out of the hole again can be made exceedingly small. The radiation coming out of this hole is then very close to the equilibrium blackbody electromagnetic radiation corresponding to the oven temperature. Wien found that the radiative energy dW per wavelength interval d has a maximum at a certain wavelength m and that the maximum shifts to shorter wavelengths as the temperature T is increased, as illustrated in the figure below. .. Wien's law of the shift of the radiative power maximum to higher frequencies as the temperature is raised expresses in a quantitative form commonplace observations. Warm objects emit infrared radiation, which is felt by the skin; near T = 950 K a dull red glow can be observed; and the color brightens to orange and yellow as the temperature is raised. The tungsten filament of a light bulb is T = 2,500 K hot and emits bright light, yet the peak of its spectrum is still in the infrared according to Wien's law. The peak shifts to the visible yellow when the temperature is T = 6,000 K, like that of the Sun's surface. .. Both these relationships were synthesized by physicist Max Planck into what is called Planck's curve. All objects emit energy in the shape of Planck's curve, where the amount and the peak energy vary only as the temperature of the body. |
.. |
.. The problem with Planck's curve is that it does not agree with Rutherford's model of the atom. Atoms absorb and emit light through a process called scattering. Photons fly near the atoms and are deflected. Sometimes their motion pushes the atom (where push means electromagnetic forces), and the photon loses energy (i.e. becomes redder). Sometimes the atom pushes the photon, and the photon gains energy (i.e. becomes bluer). However, given the nature of atoms, the photons should receive more energy than the atoms, so there should be more and more blue photons, but clearly the Planck curve drops off at short wavelengths. This is called the UV catastrophe, which was resolved by quantum physics. |
|
The fact that the temperature and energy
generation of an object can be determined by its Planck curve made the
study of spectrum the key component to stellar astronomy. The amount of
energy emitted from stars is determined by measuring their brightness or
the amount of light they emit. This is called photometry. However, two
major developments expanded our understanding of the chemical make-up of
stars. They were:
.. The invention of the spectroscope, a device that separates white light into component colors called a spectrum. And the discovery that elements emit a unique spectrum, i.e. produce a unique chemical fingerprint in the spectrum. .. The two discoveries combined to produce a new field called spectroscopy, and allowed astronomers to measure the chemical composition of stars for the first time. |

.. |
.. The discovery of spectra lines was made by Fraunhofer who, in the early 1800's, magnified the Sun's spectrum and discovered dark lines which could be identified with particular elements (based on spectra in the laboratories). |

|
.. English astronomer Lockyer, in the late-1800's, discovered an unknown element in the Sun, i.e. a set of spectral lines which did not correspond to elements in the lab. He named this element helium (Latin for Sun element). |

.. |
.. By the 20th century, spectral lines for all the elements in the periodic table have been classified and astronomers could examine the chemical composition of stars and planets. What was missing was an explanation for why these lines should exist. Why did particular atoms absorb photons at particular wavelengths? |
|
The first clue to the origin of spectral
lines was found by Kirchhoff who, in the mid-1800's, developed the three
laws of spectroscopic analysis. Gustav Kirchhoff (b. March 12, 1824,
Knigsberg, Prussia [now Kaliningrad, Russia]--d. Oct. 17, 1887, Berlin,
Ger.), German physicist who, with the chemist Robert Bunsen, firmly
established the theory of spectrum analysis (a technique for chemical
analysis by analyzing the light emitted by a heated material), which
Kirchhoff applied to determine the composition of the Sun.
.. In 1847 Kirchhoff became Privatdozent (unsalaried lecturer) at the University of Berlin and three years later accepted the post of extraordinary professor of physics at the University of Breslau. In 1854 he was appointed professor of physics at the University of Heidelberg, where he joined forces with Bunsen and founded spectrum analysis. They demonstrated that every element gives off a characteristic colored light when heated to incandescence. This light, when separated by a prism, has a pattern of individual wavelengths specific for each element. Applying this new research tool, they discovered two new elements, cesium (1860) and rubidium (1861). .. Kirchhoff went further to apply spectrum analysis to study the composition of the Sun. He found that when light passes through a gas, the gas absorbs those wavelengths that it would emit if heated. He used this principle to explain the numerous dark lines (Fraunhofer lines) in the Sun's spectrum. That discovery marked the beginning of a new era in astronomy. .. In 1875 Kirchhoff was appointed to the chair of mathematical physics at the University of Berlin. Most notable of his published works are Vorlesungen ber mathematische Physik (4 vol., 1876-94; "Lectures on Mathematical Physics") and Gesammelte Abhandlungen (1882; supplement, 1891; "Collected Essays"). |
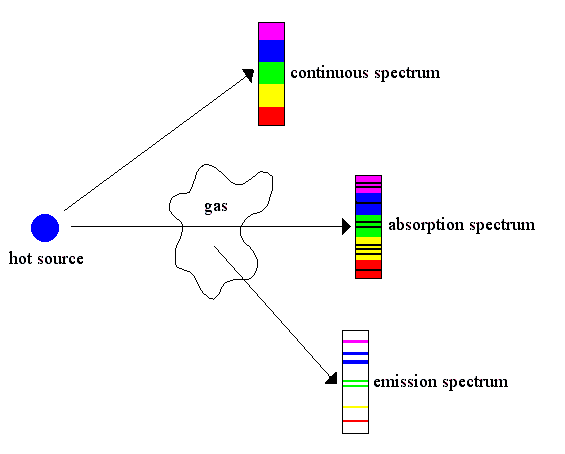
|
.. There remains a problem with the spectra and the Rutherford model of the atom. Rutherford atoms can absorb or emit photons at any wavelength because the orbit of the electron can take on any distance from the nucleus. However, the spectral lines indicate that there are preferred orbits for the electrons, that they have certain discrete distances from the nuclei. This `quantization' of electron orbits leads to the next great breakthrough in modern physics, quantum mechanics. |